Magnetic Properties of Minerals
By Paul Fears | 10 August 2020
Magnetic separation is a key stage in many mineral processing plants. Understanding the mineralogy of the deposit, and how different minerals behave in a magnetic field, is important when assessing the feasibility of using magnetic separation.

The behaviour minerals exhibit in a magnetic field is classed under one of five headings:
- Diamagnetic;
- Paramagnetic;
- Ferromagnetic;
- Anti-ferromagnetic;
- Ferrimagnetic;
These classifications define whether it is possible to separate one or more minerals from another using a magnetic separator.
Source of Magnetic Properties
The magnetic properties of any mineral reside in the electrons of the atoms or ions. In accordance with the principles of wave-mechanics, the electron, moving in a closed path about the nucleus, is considered as a current behaving like a wave, and this moving current generates a magnetic field. When a crystal is placed in an external non-uniform magnetic field, there is a force working to align the magnetic fields of the atoms to produce a magnetic moment for the whole crystal. The magnetic susceptibility χ, is the ratio of the resulting magnetic moment, M, to the strength of the external field, H.
χ = M/H
Diamagnetism and Paramagnetism
Diamagnetic minerals have a small negative value of χ and are slightly repelled by the magnetic field. In contrast, paramagnetic minerals have a small positive value of χ and are weakly attracted by the field.
Paramagnetism is associated with the spins of the electrons, whereas diamagnetism is related to their distribution in space. Diamagnetism is a property possessed by all atoms. However, when the atom contains an odd number of electrons, or has incomplete electron shells (as in the transition elements) imbalance of the electron spins causes the paramagnetic effect to overshadow the diamagnetic part of the total magnetic susceptibility. Paramagnetism is also found in metals where there is a cloud of free conduction electrons.

This behaviour is only applied generally to crystals because the internal crystal field, as a whole modifies, the magnetic effects. The electronic energy levels in a crystal are described as being split and the total magnetic susceptibility depends on the distribution of the electrons in the different levels. Therefore, in complex compounds, it is not possible to predict the magnetic properties.
In minerals, iron-bearing structures are paramagnetic. However, there are paramagnetic minerals without iron.
The differences in magnetic susceptibility are sufficient to enable separation using high-intensity magnetic separators.
Bismuth is the only one example of a diamagnetic mineral. Paramagnetic minerals are more widespread and include Hematite and Franklinite.
Ferromagnetism
Ferromagnetic minerals possess a magnetic moment even in the absence of an applied magnetic field. They are strongly attracted by even a weak magnetic field and remain permanently magnetised. However, ferromagnetic substances also exist in unmagnetised conditions when, at ordinary temperatures, the electronic magnetic moments are permanently in alignment as a result of interaction between neighbouring atoms.
Iron, Cobalt, Nickel and Pyrrhotite are typical examples of ferromagnetic minerals.
Antiferromagnetism and Ferrimagnetism
The way in which electrons align in certain crystals produces either an antiferromagnetic or ferrimagnetic effect.
Antiferromagnetism
This occurs when adjacent atoms interact in a manner to align the spins in parallel but opposed directions called antiparallel spins. The two sets of moments cancel one another and there is no permanent magnetic moment. Typical examples include metals such as Chromium and oxides such as Nickel Oxide (NiO).
Ferrimagnetism
Antiparallel alignment, in which the components in opposite directions are not equal, results in a permanent moment which is ferrimagnetism. Magnetite is a important example of a ferrimagnetic mineral.
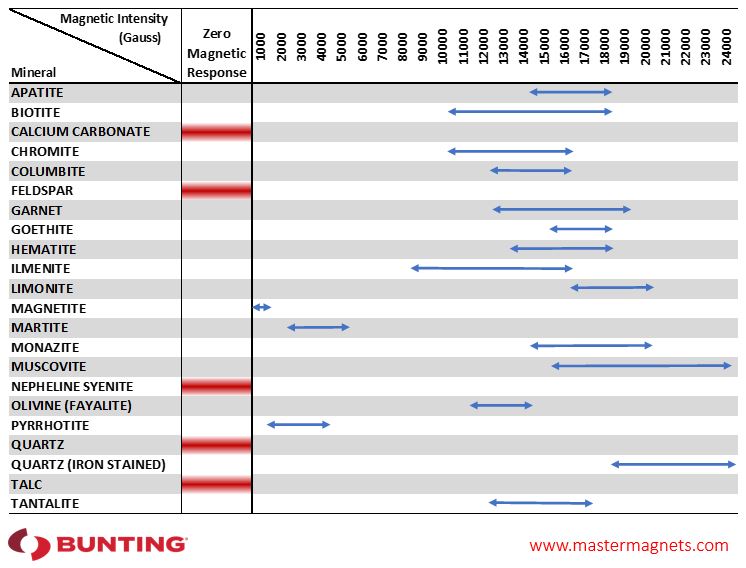
Magnetic Separation of Minerals
Understanding the magnetic properties of the minerals in a particular deposit is vital when optimising magnetic separation. The wide range of magnetic separator designs use different strengths and types of magnetic field to produce a separation. Several designs of magnetic separator may feature in a single mineral processing operation.

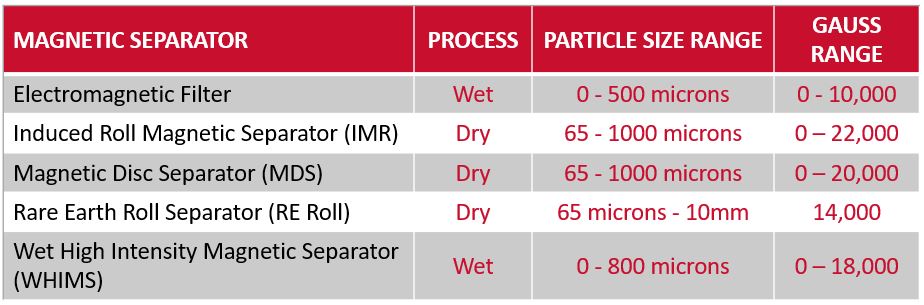
The most commonly used high-intensity magnetic separators in the mineral processing industry are:
Dry Processing
Wet Processing
Separation is commonly optimised with controlled tests undertaken at Bunting’s Centre of Excellence in the United Kingdom.
Related Mineral Processing Technical Articles
Magnetic separators – Mineral Processing Laboratory
As traditional mineral reserves are depleted and more complex deposits are mined, understanding the mineralogy in terms of magnetic susceptibility is vitally important. Bunting engineers work closely with mineral processors and plant designers, often at the project feasibility stage, providing advice on the optimum design of magnetic separator and stage within the process.
Bunting-Redditch has one of the most comprehensive magnetic separation mineral processing testing laboratories in the world. Their Laboratory Technicians have decades of experience in mineral processing. Controlled tests ensure that the most suitable and cost-effective machinery is recommended for each application. The laboratory is equipment with a wide range of equipment including:
- Smaller scaled versions of industrial Magnetic Separators. This equipment is used to accurately scale up to industrial capacities and calculate performance guarantees;
- X-Ray Fluorescence and X-Ray Diffraction analysis are available for chemical assay and mineralogical identification to aid the development of a viable process route for each application;
For further information on our range of magnetic separation equipment designed for purifying ceramics and non-metallic minerals, or to arrange sample tests in our laboratory, please contact us on:
Email: Gordon Kerr at GKerr@buntingmagnetics.com
Telephone: +44 (0) 1527 65858
To keep up to date with our news and technical reports, please follow us on social media